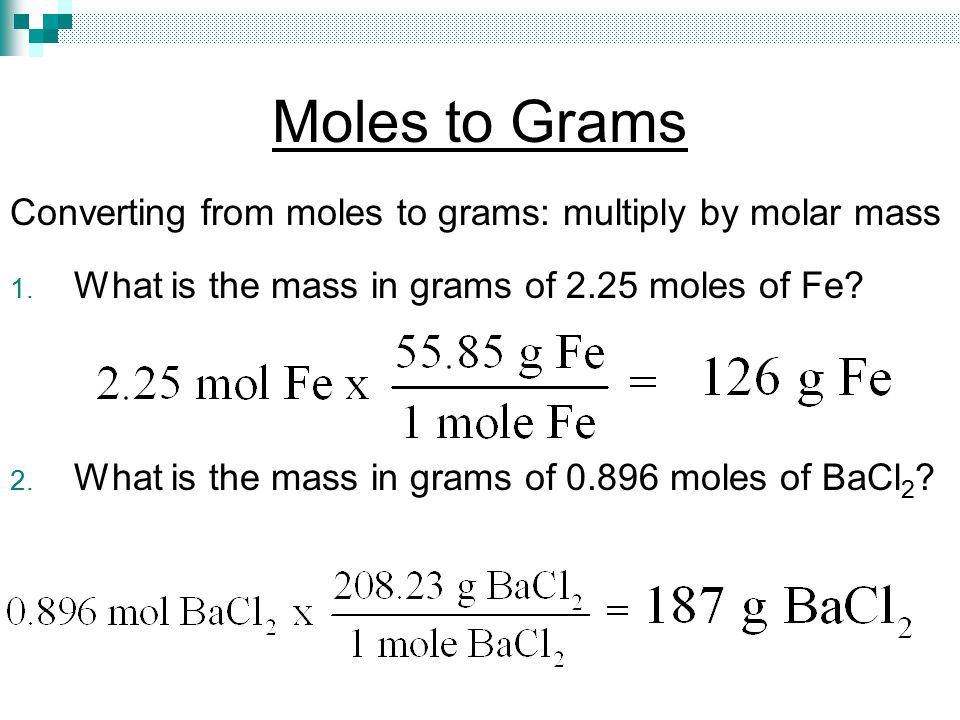
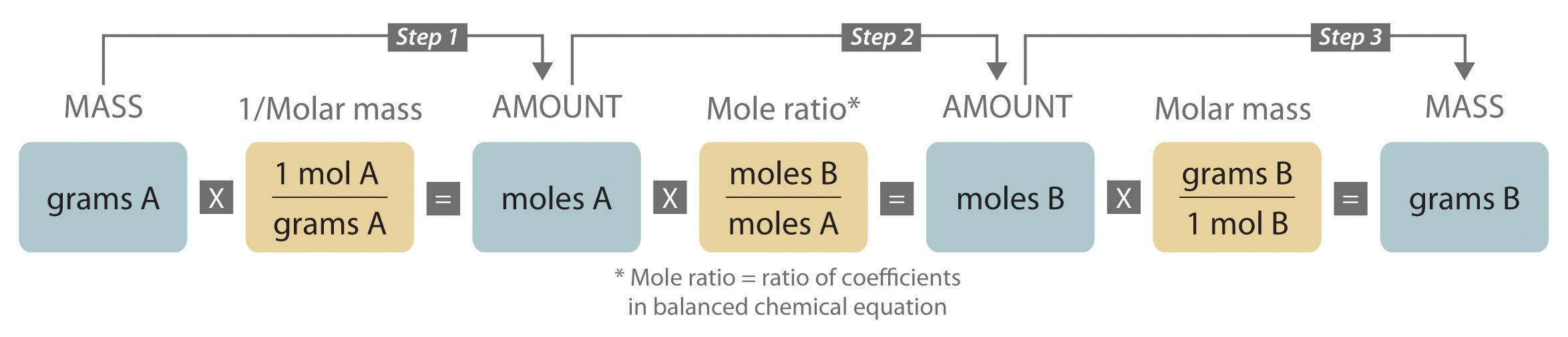
By Part of In a general chemistry class, you usually end up having to perform a lot of conversions involving moles (mol). Whether you’re converting from moles to grams, moles to volume, or moles to particles (atoms or molecules), use this quick guide to remind you of how to do each type of mole conversion: • Converting from mass (grams) to moles: Divide your initial mass by the molar mass of the compound as determined by the periodic table.
The Dalton (or atomic mass unit (amu) ) is a unit of mass defined as 1/12 weight of carbon-12 atom in ground state. 1 Da = 1/12 m(12C) The number of atoms in 1 mole. Apr 19, 2017. To calculate the mass of a single atom, first look up the atomic mass of carbon from the Periodic Table. This number, 12.01, is the mass in grams of one mole of carbon. One mole of carbon is 6.022 x 1023 atoms of carbon (Avogadro's number). This relation is then used to 'convert' a carbon atom to grams. The molar mass of an element is the mass, in grams, of a mole of atoms for each element. Avogadro's Number: converts between moles and molecules/ions/atoms Stoichiometry Flowchart & Chemical Conversions. Autobiography Of Narendra Modi Pdf Free Download. So if you have x grams of a substance, and the molecular weight is y, then the number of moles n.